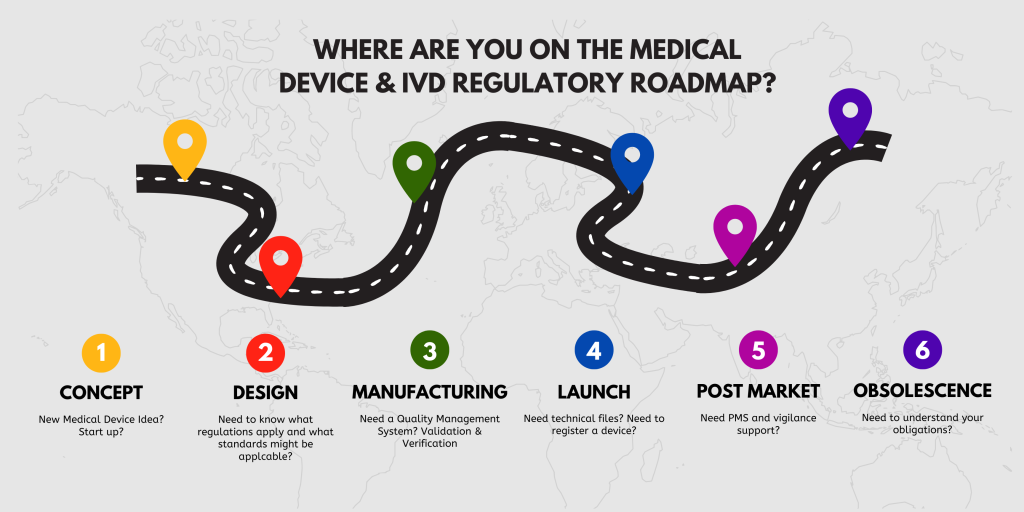
1. Concept
Do you have a medical device or IVD idea? Are you a medical device start up? If so the prospect of medical device and IVD regulations can be daunting, especially if you have not done it before. Using a medical device consultant is usually a good approach to take. Using their expertise is vital in understanding what you need in planning your regulatory pathway, and making the journey as smooth and painless as possible. Patient Guard is a well respected and market leading medical device and IVD regulatory consultancy, established in 2017. Our experts cover all types of medical device from class I to Class III and IVDs from Class A to Class D, we have grown to be a one stop shop for everything you need on your medical device or IVD compliance journey.
See our blog on:

1. Concept - How we can help you?
Product Determination
Is your product a medical device or IVD? Sometimes it can be difficult to know if it falls within the definition of a medical device or IVD. Especially with Software apps, combination products, creams and topical products. We have the experience you need to help determine if your products are medical devices or IVDs.
Device Classification
Once it has been established that your product is a medical device or IVD, the next step is identifying its classification. in the EU and UK, Is the medical device a class I, Class Is, Class Im, Class IIa, Class IIb or Class III medical device? Is the IVD Class A, B, C or D? In the FDA is there a predicate device that identifies what class your device falls into?
Our seasoned medical device consultants and IVD consultants can help you with classification, this is very important as this will determine the regulatory pathway that needs to be taken. Getting this wrong can be costly.
2. Design & Development
Once you have come up with the medical device or IVD concept, its time to start design and development. Its important that this is done carefully and is fully documented. Design history is an important part of the technical data.
See our blog on:

Enquire for more information
2. Design & Development - How can we help you?
Regulatory Requirements
Understanding which regulatory requirements are relevant to your medical device or IVD is essential. Depending on what type of device or IVD it is will depend on what requirements are needed to be demonstrated that compliance has been met. If your medical device or IVD has user contact, then you may need to prove its biocompatibility. if the device is electronic then you may need to demonstrate electrical safety and so on.
Performing this during the design and development stage is critical to understand how the device may need to be designed or consider other factors. Patient Guard as a medical device consultancy has assisted hundreds of clients over the years in reviewing the regulatory requirements for their medical device and IVDs. Doing this at this stage in the development process will allow you to plan costs, project length etc.
Applicable Standards
The main way to demonstrate conformity to the regulatory requirements is to use consensus or harmonised standards. These are usually international or regional standards that specify how something should be either designed, manufactured, tested and validated.
There are thousands of standards, many manufacturers of medical device and IVDs may not know which ones are applicable to them when designing a medical device or IVD. By using Patient Guard’s expertise we can help you identify applicable standards, and provide information on which part of the standard may or may not be applicable to your medical device or IVD.
3. Manufacturing
Once the design has been finalised its time to transfer to manufacturing. This can be challenging and may require design changes to ensure that the device can be scaled up for manufacturing. Its important that manufacturing processes including any processing additives are considered carefully and fully documented. All manufacturing processes need to be verified and validated. All of these processes, work instructions and records should be kept within a QMS.
Product verification and validation should always be done on the finished manufactured product. Not on the prototype of the device during the design and development stages.
See our blog on:
3. Manufacturing - How can we help you?
Quality Management
Implementing a Quality Management System (QMS) that is compliant with regulatory requirements is essential. In the UK and the EU it is recommended that you implement an ISO 13485 QMS. In the USA you need to implement a QMS in line with the FDA CFR 21 part 820, this is called the QSR. Our Quality Assurance consultants have assisted with the implementation of over 40 Quality Management Systems. All of these systems have been assessed by Notified Bodies and certification bodies with a 100% success rate.
Biological Evaluation
If your medical device or IVD has contact with the user, then it will need to be assessed for its biocompatibility against the ISO 10993 series of standards. This is a key requirement in the EU under MDR 2017/745 / IVDR 2017/746, in the UK under UK MDR 2002 and by the FDA.
Patient Guard’s toxicologists can provide you with biological evaluation plans (BEP), these assess the device based on the materials used in the device and their potential risk, the plan will detail if additional testing is required. Patient Guard can also write Biological Evaluation Reports (BER) which summarise the findings of risk mitigation and testing to prove that the devices are biologically safe.
We have written over 300 BEPs and BERs for clients since we started in 2017.
4. Launch
Before you launch your medical device or IVD, it is important to ensure that you have met all of your regulatory obligations before placing product on the market. Have you:
- Verified and Validated the device?
- Verified and Validated the packaging?
- Verified and Validated Transport, Storage, Handling?
- Performed Biological Evaluation?
- Performed Usability Engineering?
- Performed Clinical Evaluation?
- Correctly Labelled the device?
- Provided Instructions for Use?
- Created Technical Files (EU/UK)?
- Got a PRRC in place (EU)?
- Got an UKRP in place (UK)?
- Got a EUAR in place (EU)?
- Have a QMS in place and operating it?
- Contracted a Notified Body (EU/UK)?
- Registered the device?

Enquire for more information

4. Launch - How can we help you?
Medical Writing
Clinical Evaluation of medical device and Performance Evaluation of IVDs is an essential component of demonstrating compliance with regulations. Our medical writers can support you with writing clinical evaluation plans (CEP), these are written reports that include information on how you are going to demonstrate that the device based on its clinical intended use meets safety and performance and applies the ‘state of the art’. This can be through supporting clinical publications and literature, through performing clinical studies and assessing the risk management of the device. Patient Guard’s medical writers can then provide a critical analysis of all this evidence in a Clinical Evaluation report (CER), summarising all of the data generated to show that the clinical benefit of using the device out weighs the risks associated with the use of the device.
Technical Files
In the UK and the EU technical documentation needs to be created in the form of a technical file, sometimes also called medical device files. These files contain all the information gathered and generated by the manufacturer of medical devices and IVDs. These files are required for all classes of medical devices and IVDs. Each medical device or IVD group require their own technical file. The files need to be generated following a specific format. The STED (Summary of Technical Documentation) format, this format is required to produce technical files that are uniformed in approach. As these files are reviewed by Notified Bodies and regulatory authorities. Following this file format allows everybody to understand how and where to find the relevant data within the files.
Here at Patient Guard we are experts at creating and maintaining Technical Files for our customers, ensuring that they meet compliance regulatory requirements and demonstrating to Notified Bodies and Regulatory Authorities that the manufacturer has generated all the necessary documentation required by the regulations. With over 100 Technical Files written by our medical device consultants since 2017 Patient Guard has the experience you need, to get it right the first time around.
510k - Documentation
The FDA require documentation as part of a 510k submission to be very precisely written and organised. It is important that you ensure you use an expert when generating a 510k submission. The FDA will not accept any mistakes or files that do not follow their strict criteria. Luckily, Patient Guard has experienced professional consultants on hand to help create these files for you.
Person Responsible for Regulatory Compliance (PRRC)
Having a PRRC is a new requirement in the EU under the MDR and the IVDR. In article 15 of both regulations, it details the requirements and qualifications needed to be a PRRC. Manufacturers of medical device and IVDs must have a PRRC at their disposal. For Micro companies and SME’s the PRRC can be outsourced to a service provider. Patient Guard meets all the necessary requirements and qualifications to act as your PRRC.
Notified Body Mock Audits
Notified Body audits can be a daunting prospect. Especially with the high costs involved in terms of audit days and other review costs that the Notified Body charges. If your documentation is not correct or doesn’t follow the expected format then non-conformances can be raised. This can lead to additional audit days and reviews and can become a costly exercise.
At Patient Guard we can help you prepare for Notified Body audits. We can talk you through what is to be expected, we can be there with you on the day for support and we can perform mock Notified Body audits, to rigorously test your QMS and Technical Files before audit day. Giving you the best possible chance to pass first time round.
UK Responsible Person
For medical device and IVD manufacturers who are not UK based but place devices on the UK market, a UK Responsible Person (UKRP) is required.
The UKRP is responsible for registering the manufacturers medical device and IVDs with the MHRA.
As a dedicated UKRP with a UK registered business, Patient Guard Ltd is well placed to help you gain UK market access for your medical device and IVDs.
EU Authorised Representative
For medical device and IVD manufacturers who are not EU based but place devices on the EU market, an EU Authorised Representative (EUAR) is required.
The EUAR is responsible for registering the manufacturers medical device and IVDs with a Competent Authority based in the EU.
As a dedicated EUAR with a German registered business, Patient Guard Europe UG, is well placed to help you gain EU market access for your medical device and IVDs.
5. Post Market
Monitoring a medical device and IVD is important once it has been launched, to ensure that any unforeseen risks are captured and mitigated.


Enquire for more information
See our Blog on:
5. Post Market - How can we help you?
Post Market Surveillance
Once your medical device is launched and registered, as a manufacturer you have additional regulatory obligations. This is in the form of post market surveillance (PMS). This is monitoring data generated from things like; customer feedback, customer complaints, competitor data, regulatory updates, updates to standards that may affect the medical devices or IVDs placed on the market, new guidance documents issued by authorities etc.
Manufacturers are obligated to generate Post Market Surveillance Reports (PMSR) (class I medical devices) or Periodic Safety Update Reports (PSURs) for class IIa, class IIb and class III medical devices.
This data as well as data generated in relation to PMCF is required to be included in any clinical evaluation report updates.
Patient Guard as a medical device consultancy can hold your hand through these processes and help create the necessary reports needed, as well as update your clinical evaluation reports.
PMCF
Medical Devices placed on the market now require manufacturers to generate real world data. This is to demonstrate that the devices are performing as intended, are being used as intended and remain ‘state of the art’.
Post Market Clinical Follow-up (PMCF) is now expected for nearly all types of medical device placed on the market and there is a strengthened emphasis from regulatory authorities on this type of data being generated.
Patient Guard as a medical device consultancy can help you generate robust PMCF plans, help to produce PMCF surveys and once the data has been gathered, produce PMCF reports. PMCF reports must be included as clinical evidence in the clinical evaluation reports, which Patient Guard can also assist you with.
Vigilance
Medical device and IVD vigilance is an important part of post marketing activities. Vigilance relates to the reporting of ‘reportable’ adverse events to the regulatory authorities and Notified Bodies (if applicable). Within the regulations are requirements for manufacturers to report to strict timescales and investigate the reported incident. The manufacturer may need to initiate corrective actions based on the out come of the investigation, this could include design changes, manufacturing changes, material changes, or even pulling the device from the market. Manufacturers may need to issue Field Safety Notices (FSN) as part of their Field Safety Corrective Actions (FSCA).
This can be a tricky situation and its important that you seek expert advice if this happens. Reporting in the correct way is vital and working with the authorities essential.
Patient Guard as a medical device consultancy can assist you in all aspects of vigilance and guide you through the process.
QMS Internal Audits
In maintaining a QMS it is an essential requirement that internal audits are performed. This is to ensure that the system is working as intended, and to check if the system is fit for purpose. Notified Bodies and certification bodies now check that internal audits are being performed in a non-bias way. This is difficult for small organisations to achieve due to the smaller workforce, it is likely that the auditor will know the auditee, this can also lead to bias in the audit, the auditor may not want to ‘upset’ the auditee with any areas found to be out of conformance. It is now recommended that internal audits are outsourced to a 3rd party with no attachment to the organisation.
Our internal auditors are fully trained and experienced in ISO 13485, and can perform internal audits for your organisation. Our flexible approach allows internal audits to occur with minimal disruption to your business.
6. Obsolescence
Eventually all good things come to an end, medical devices and IVDs are no exception. Once a device has passed its usefulness it might need to be made obsolete. However, this isn’t as simple as just stopping selling the device, there are some regulatory requirements to consider in doing so.

6. Obsolescence - How can we help you?
Obsolescence Planning
Sometimes medical device and IVDs can become old and need to be retired, this might be because there is a new better device or the device isn’t selling as well as it once did. Manufacturers may decide to stop selling a medical device for many reasons. However, there are lots of things to consider when you decide to stop selling a medical device. Such as will there still be support for customers who are still using the devices? How long do you need to keep records for? How do you deal with any adverse events that may occur with product still on the market?
Patient Guard can help you plan and advise on what requirements you may need to comply with when retiring a medical device or IVD.
Why Choose Patient Guard:
- Expertise and Experience: Patient Guard as a medical device consultancy boasts a team of seasoned professionals with in-depth knowledge of global regulatory standards. Leveraging our over two decades of experience, we have not only amassed valuable insights but have also successfully guided numerous clients through the regulatory maze, ensuring compliance and peace of mind.
- Tailored Solutions: Recognizing the unique nature of every Medical Device, our approach is highly customized. We meticulously tailor our services to meet the specific needs of your products and organization, ensuring a personalized and effective regulatory strategy.
- End-to-End Support: At Patient Guard, we believe in offering comprehensive support that spans the entire product lifecycle. From initial consultations to ongoing compliance management, we are your dedicated partners, providing unwavering support and expertise. Rest assured, our commitment to you ensures sustained regulatory adherence and success.
- Timely and Efficient: We recognize the paramount importance of time in the competitive landscape. Patient Guard prides itself on delivering timely and efficient services. Our streamlined processes help you expedite the regulatory journey without compromising on quality, allowing you to bring your innovative healthcare solutions to the market swiftly and confidently.
In choosing Patient Guard as your Medical Device Regulatory & Quality Assurance Partner, you’re not merely selecting a service provider; you’re opting for a dedicated ally committed to your success. Let us expertly navigate the complexities of regulations, allowing you the freedom to focus on what you do best – innovating and improving healthcare solutions for the world.
What they say about us
ExcellentBased on 5 reviews
 Hannah Maddison2024-03-28Fantastic, knowledgeable team that are always there to help. My appointments have always been booked in very promptly and have always ended with all my queries resolved. I have found the team very flexible and their breadth of knowledge is second to none. Patient Guard are without doubt my go-to for all the regulatory aspects of my medical device role.
Hannah Maddison2024-03-28Fantastic, knowledgeable team that are always there to help. My appointments have always been booked in very promptly and have always ended with all my queries resolved. I have found the team very flexible and their breadth of knowledge is second to none. Patient Guard are without doubt my go-to for all the regulatory aspects of my medical device role. Richard Crow2024-02-21Patientguard are an excellent source of Medical regulatory compliance advice, we have taken advantage of their various services from their EU Rep service, to helping with Technical Files all the way through to using their ISO Templates to implement our ISO 13485 system.
Richard Crow2024-02-21Patientguard are an excellent source of Medical regulatory compliance advice, we have taken advantage of their various services from their EU Rep service, to helping with Technical Files all the way through to using their ISO Templates to implement our ISO 13485 system. George Kitching2024-02-19David Small and PatientGuard have been extremely helpful and supportive in assisting us with producing and updating our Technical File and Appendices for MDR certification.
George Kitching2024-02-19David Small and PatientGuard have been extremely helpful and supportive in assisting us with producing and updating our Technical File and Appendices for MDR certification. Tracey Slater2024-02-19Patient Guard have been a great support service to Cormed, providing help and advice promptly when ever requested. They have become a virtual department within Cormed enabling us to keep up to date and comply with the regulatory requirements whilst ensuring our QMS works for us at the same time.
Tracey Slater2024-02-19Patient Guard have been a great support service to Cormed, providing help and advice promptly when ever requested. They have become a virtual department within Cormed enabling us to keep up to date and comply with the regulatory requirements whilst ensuring our QMS works for us at the same time. Peter Reeve2024-02-18STEPPER design, manufacture & distribute eyewear across the globe. With the increasingly complex landscape concerning the placing of Mecial Devices onto the market, we realised we needed professional guidance. We found Patient Guard via a simple internet search and are delighted we did! They provide a pragmatic solution to our needs, are totally reliable & always available to answer our (often simplistic) questions. They are highly efficient & responsive to what is a changing picture in our world and nothing is too much trouble. We have a much better understanding of regulatory affairs and our responsibilities as manufacturers & distributors and they support us in navigating the requirements in different territories. Updating our Declaration of Conformity, ensuring our labelling is compliant and acting as our PRRC are the key areas of their service for us.
Peter Reeve2024-02-18STEPPER design, manufacture & distribute eyewear across the globe. With the increasingly complex landscape concerning the placing of Mecial Devices onto the market, we realised we needed professional guidance. We found Patient Guard via a simple internet search and are delighted we did! They provide a pragmatic solution to our needs, are totally reliable & always available to answer our (often simplistic) questions. They are highly efficient & responsive to what is a changing picture in our world and nothing is too much trouble. We have a much better understanding of regulatory affairs and our responsibilities as manufacturers & distributors and they support us in navigating the requirements in different territories. Updating our Declaration of Conformity, ensuring our labelling is compliant and acting as our PRRC are the key areas of their service for us.
Patient Guard news letters