FDA - 483 Warning Letters
"FDA Form 483," is a document issued by the U.S. Food and Drug Administration (FDA) to a company following an inspection. It outlines any observations of non-compliance or violations of regulatory requirements

FDA 483 warning letters
Every Medical Device or IVD manufacturer dreads receiving a Form 483 or Warning Letter from the US Food and Drug Administration (FDA). Officially termed a “Notice of Inspectional Observations,” the FDA 483, commonly known as a “483,” is handed out following an on-site inspection. This occurs when an FDA field investigator identifies deficiencies in your Quality Management System or conditions that breach the Food, Drug, or Cosmetic Act.
Receiving a 483 or Warning Letter can have serious repercussions for manufacturers. Consequently, it is essential to maintain a robust Quality Management System and adhere to regulatory requirements. Staying proactive and vigilant in ensuring compliance helps manufacturers avoid such situations and maintain their reputation in the industry.

How to deal with a 483 letter
After the field inspector completes their assessment, they send the final 483 report to their superiors. If the identified issues are grave or if your response to the 483 is considered inadequate, the FDA might issue a Warning Letter to your organization.
This Warning Letter serves as the FDA’s advisory, indicating substantial violations of FDA regulations. It precisely outlines the violations, emphasizing the imperative need for corrective action. You are required not only to rectify the problems but also to inform the FDA about your detailed plans for correction, outlining preventive measures to prevent similar issues from recurring. Subsequently, the FDA assesses the adequacy of your corrections to ensure compliance. It is crucial to respond comprehensively and promptly to mitigate potential consequences and uphold regulatory integrity.
How to respond to a Form 483 or warning letter
You must respond to the 483 or a Warning Letter promptly and identify your course of action to correct the findings within the FDA’s specified timeframe. A detailed response to each observation or violation noted is also required. The quality and promptness of your response to this letter are extremely important.

How can Patient Guard help?
Patient Guard is here to support you in crafting a robust response to an FDA Form 483 and Warning Letter. We offer guidance to ensure your Quality Management System aligns seamlessly with the regulations. As part of our comprehensive services, we undertake several crucial steps:
Firstly, we meticulously analyze the findings outlined in the FDA Form 483 and/or Warning Letter. Subsequently, we assist in devising an appropriate timeline to meet the FDA’s requirements, guiding your company in charting a clear course of action. Our team proposes specific “Corrective Actions” tailored to your quality system, ensuring they align with regulatory expectations.
Moreover, we actively assist in implementing these corrective actions, responding effectively to the issues raised in the FDA Form 483. Throughout this process, we remain readily available to address any queries, whether from your end or the FDA’s, ensuring smooth communication and resolution.
Patient Guard has a proven track record of collaborating with Medical Device and IVD companies, assisting them in formulating precise and compliant responses to FDA 483 and Warning Letters. Rest assured, our expertise will guide you toward a resolution that satisfies regulatory requirements and upholds the integrity of your organization.
Key Benefits of Partnering with Patient Guard:
- Expertise: Our team comprises seasoned professionals with extensive experience in regulatory compliance and quality assurance within the healthcare industry. We bring unparalleled expertise to every audit, ensuring thoroughness and accuracy in our assessments.
- Tailored Approach: We understand that every organization has unique compliance needs and challenges. That’s why we customize our internal auditing services to address your specific requirements, mitigating risks and maximizing efficiency.
- Regulatory Compliance: By staying abreast of evolving regulatory requirements and industry best practices, we help you maintain compliance with ISO standards, FDA regulations, and other relevant guidelines.
- Continuous Improvement: Internal audits are not just about achieving compliance; they’re also about driving continual improvement. We provide actionable insights and recommendations to enhance your quality management processes and optimize performance.
- Peace of Mind: With Patient Guard as your partner, you can have confidence in the integrity and reliability of your quality management system. Our rigorous auditing practices and commitment to excellence give you peace of mind, allowing you to focus on innovation and growth.
Summary
Patient Guard, your trusted partner in regulatory compliance and quality assurance consultancy. Our services are tailored to ensure your organization’s adherence to stringent regulatory standards, including ISO 13485, ISO 9001, and FDA Quality System Regulation (QSR). With our expertise and meticulous approach, we help you navigate the complex landscape of medical device regulations, mitigating risks and enhancing operational efficiency. Explore our comprehensive solutions designed to elevate your compliance standards and bolster your reputation in the healthcare industry.

Tracey Slater, Cormed
Find out more about medical device compliance and regulations
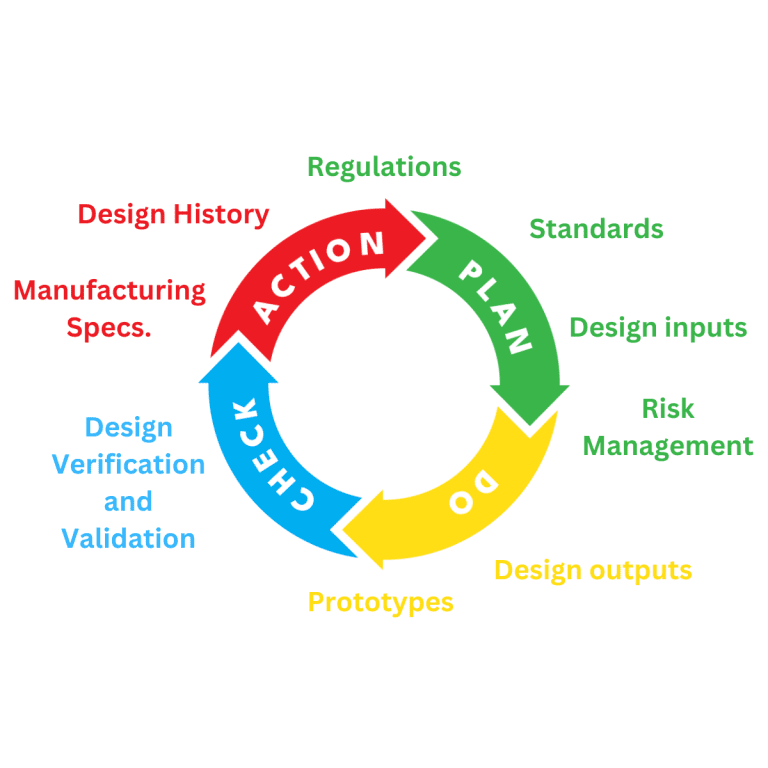
Medical Device Design and Development
Planning for the design and development of a medical device is a requirement of regulatory systems. All manufacturers of Medical

Understanding the core elements of Quality Management Systems
In today’s competitive marketplace, delivering high-quality products and services is essential for business success. A Quality Management System (QMS) is

5 Differences Between ISO 13485 & FDAs Medical Device QSR
Medical device manufacturers face a daunting task: navigating complex regulatory landscapes to ensure their products meet safety and quality standards.
ISO 13485 Standard – Quality Management System
The ISO 13485 standard was built around the structure of ISO 9001 which is a standard for Quality Management Systems


