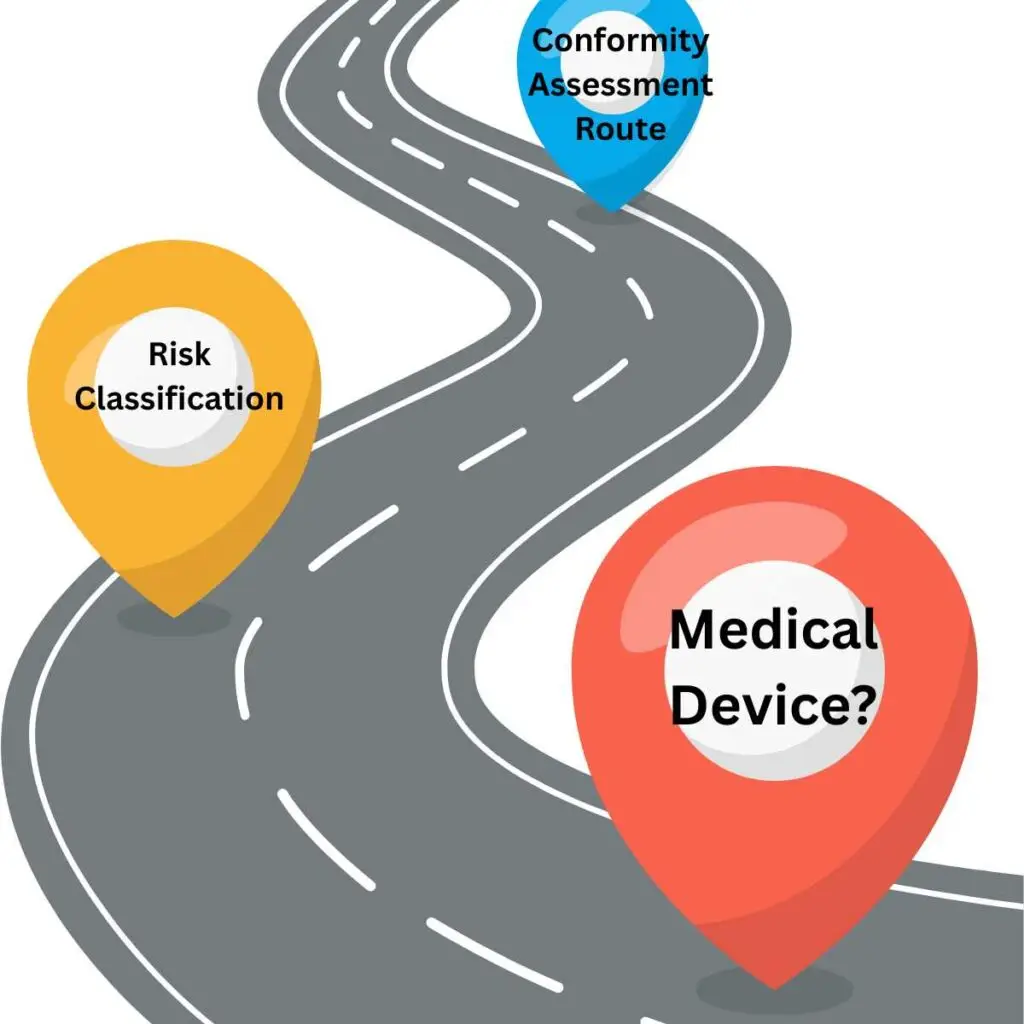
Navigating the EU Medical Device Regulatory Roadmap: A Guide for Manufacturers
Bringing a medical device to market in the European Union is a complex process. With the implementation of the EU Medical Device Regulation (MDR) 2017/745, manufacturers must follow a detailed regulatory roadmap to achieve CE marking and ensure continued compliance.