Quality Assurance (QA) vs Quality Control (QC) is a commonly asked question but both are important aspects of Quality Management Systems (QMS). They work side by side but the two are defined differently. It is common for these two terms to be used interchangeably, however, there are distinctive differences between the two. QA manages the quality whereas QC verifies it.
The QMS is the system that connects QA and QC together, with QA sitting within the QMS as a larger aspect, then within QA you will find an element of QC. (See figure 1 below).
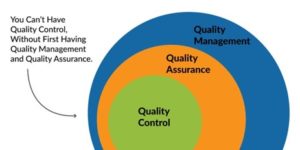
The relationship between QMS, QA and QC.
Definitions
QA can be defined as “part of quality management focused on fulfilling quality requirements.” While quality assurance relates to how a process is performed or how a product is made, quality control is more the inspection aspect of quality management. An alternate definition is “the operational techniques and activities used to fulfil requirements for quality.”
QC can be defined as “part of quality management focused on providing confidence that quality requirements will be fulfilled.” The confidence provided by quality control is twofold—internally to management and externally to customers, government agencies, regulators, certifiers, and third parties. An alternate definition is “all the planned and systematic activities implemented within the quality system that can be demonstrated to provide confidence that a product or service will fulfil requirements for quality.”
List 1. Quality Assurance vs Quality Control
5 Key Differences Between QC and QA
Quality Control
• Promotes corrective actions
• Focuses on products
• Focuses on detecting issues and correcting them
• Reviewing the quality of all factors involved in production
• Implementing statistical techniques and software to monitor and measure product conformity
Quality Assurance
• Promotes preventative actions
• Focuses on Processes and Procedures
• Aims to improve processes to prevent things from going wrong in the future
• Prevent defects, non-conformances, and errors
• Ensuring standards are being followed
Quality Assurance vs Quality Control during software testing.
Definition of Verification and Validation:
• QA focuses on verification of software, ensuring it meets the required specifications which is the essential focus for producing high quality performing software.
• QC on the other hand focuses on software validation, this would bring more focus onto whether or not the specification captures the end users’ requirements.
Statistical techniques used in Quality Assurance and Quality Control:
• During QA related testing activities, the statistical technique applied is Statistical Process Control (SPC). SPC tools and procedures can help you monitor a processes performance, detect live issues in internal systems and can also assist with finding solutions for production issues.
• During QC related testing activities, the statistical technique applied is Statistical Quality Control (SQC). This technique involves acceptance testing which monitors and measures the quality of the software via specific data sets. It may involve producing graphical data and control charts to decide whether or not a process should carry on as is or highlight the potential need for adjustments to be made.
Quality Assurance and Quality Control in software development testing:
• QA is responsible for full software development life cycle. QA defines the processes, policies, methods and procedures, formulate strategies, create checklists, plans, and ensures any applicable standards and regulations are being followed. All this groundwork then comes together to help prevent issues from occurring before the process has even begun.
• QC is responsible for software testing life cycle. This process follows the defined processes and procedures set out by QA to ensure the testing of software remains at a high level of quality throughout the development life cycle. High-quality QC assists in producing a software product that meets or exceeds the end users’ needs and expectations.

Quality Assurance vs Quality Control during production.
The QMS has many tools within it that assist with implementing QA during production, many of which activities happen before the product is produced as a means of preventing product non-conformances.
This includes activities such as:
• Monitoring and evaluating suppliers of raw materials/ implementing purchasing controls
• Carrying out efficient goods-in checks
• Performing supplier Evaluation Audits
• Working with approved suppliers
• Creating working instructions and standard operating procedures
• Ensuring employees are fully trained on processes and procedures
• Risk assessing the production/ work environment
• Understanding and considering the needs and requirements of the customer
• Having clear and defined product specification sheets
• Controlling the cleanliness of the work environment
• Ensuring adequate resources and infrastructure are in place and available
The QC aspect comes in once all the above measures have been implemented and the product is live in production.
One of the most common terms used in production is ‘Quality Control checks’. These checks can be carried out at any or multiple stages across production lines and may involve:
• Visual marking and labelling checks against conforming standards/ regulations
• Packaging, branding, graphics, and seal integrity
• Weight and dimension checks
• Damage to raw materials checks for scratches, dints, chips etc.
• Cleanliness checks
• Machine and equipment calibration and maintenance/ servicing
• Colour specifications
• Barcoding/ serial/ lot number/ QR code traceability and identification checks
• Electrical safety testing checks
In some instances where manufacturing is outsourced, the QC checks are established by the legal manufacturer and agreed to by both parties via a Quality Agreement.

QC checks are always made specific to the product in hand and should take into consideration the risks of the product to the end user. If a product poses many high-risk factors, then more frequent and thorough checks should be implemented.
In fast-paced production lines that produce high volumes of stock, it may not be practical for employees to keep on top of the amount of QC checks required. There are ways and means of implementing Smart manufacturing methods that are controlled and monitored via sophisticated software applications. Such applications are capable of carrying out effective QC checks, monitoring and measuring data in real time. This then allows for live trend analysis and the operators can be notified when tolerance levels are broken.

Conclusion
QC and QA have many similarities and differences. The main take away is that one can’t work effectively without the other. Within all types of industry settings these tools assist in producing high-quality products that meet or exceed customer requirements, and reduce the overall risk to the end-user.
Written by our Quality Assurance Manager
About the author

‘Laura is qualified to degree level in Forensic and Applied Science, with additional higher-level studies in microbiology and biotechnology. Since graduation she has worked across a wide range of industries with scientific-based roles in clinical healthcare, administrative, manufacturing, and educational settings and had experience working in project management and coordination roles. Laura has over 10 years’ experience working within, BRC, FSA, GMP/ GLP, ISO 9001 and UKAS accredited laboratories in multidisciplinary, research support, technical support, QC and QA roles. She now works for Patient Guard as a Quality Manager and offers consultancy services for ISO 13485 QMS Implementation/accreditation and management. Laura also provides Internal Audits and supplier evaluation auditing services for the medical device industry and is here to help you with your company’s Quality Management Representative and Patient Guard Quality services.’
References
Quality Assurance vs. Quality Control and Where Qualtrax Fits In
5 Major Differences between Quality Assurance and Quality Control Testing
https://www.softwaretestinghelp.com/quality-assurance-vs-quality-control/



