Person Responsible for Regulatory Compliance (PRRC)
The EU Medical Device Regulations and In Vitro Diagnostic Regulations have made it a legal requirement for Manufacturers to have a PRRC

- Person Responsible for Regulatory Compliance
- What is a PRRC?
- Key Responsibilities of the PRRC
- Patient Guards PRRC Service
- Patient Guards PRRC Service Includes
- Our PRRC Service Process
- Benefits of working with Patient Guard
- Cost of the PRRC Service
- Getting Started with Patient Guards PRRC Service
- PRRC FAQs
Person Responsible for Regulatory Compliance (PRRC)
The Person Responsible for Regulatory Compliance (PRRC) holds a pivotal position. Under the European Union’s Medical Device Regulation (MDR) 2017/745 and In Vitro Diagnostic Regulation (IVDR) 2017/746 article 15, the PRRC plays a critical role in ensuring adherence to regulatory standards and safeguarding patient safety.

What is a PRRC?
The PRRC is a designated individual within a medical device manufacturer’s organization responsible for overseeing compliance with regulatory requirements. Their responsibilities encompass various aspects of regulatory compliance, including ensuring conformity assessment, maintaining technical documentation, and conducting post-market surveillance activities.
Key Responsibilities of the PRRC
Conformity Assessment: The PRRC is responsible for ensuring that medical devices and in vitro diagnostic devices undergo thorough conformity assessment procedures as per the requirements outlined in MDR and IVDR. This involves assessing the product’s compliance with essential requirements, including safety and performance standards.
Technical Documentation: Maintaining comprehensive and up-to-date technical documentation is a crucial aspect of the PRRC’s role. They must ensure that all relevant documentation, such as technical files and design dossiers, accurately reflects the device’s characteristics and compliance status.
Post-Market Surveillance: The PRRC oversees post-market surveillance activities to monitor the safety and performance of medical devices and IVDs once they are placed on the market. This includes collecting and analyzing data on adverse events, conducting trend analysis, and implementing corrective and preventive actions as necessary.
Regulatory Liaison: Acting as a liaison between the manufacturer and regulatory authorities, the PRRC facilitates communication and collaboration on regulatory matters. They ensure timely submission of regulatory documentation and respond to inquiries or requests from regulatory agencies.

Patient Guards PRRC Service
At Patient Guard, we understand the complexities of medical device regulation and the critical role of the PRRC in ensuring compliance and patient safety. As a leading medical device regulatory and quality assurance consultancy, we offer comprehensive PRRC services tailored to meet the unique needs of our clients.
Our PRRC Service includes
Qualified Expertise: Our team consists of highly qualified and experienced regulatory professionals with in-depth knowledge of MDR, IVDR, and other relevant regulatory frameworks. Our PRRCs possess the necessary expertise to navigate the regulatory landscape and ensure compliance with applicable requirements.
Regulatory Compliance: We provide comprehensive support to manufacturers in achieving and maintaining regulatory compliance under MDR and IVDR. From conducting gap assessments to developing regulatory strategies and preparing technical documentation, we assist our clients at every stage of the compliance process.
Post-Market Surveillance: Our PRRCs oversee post-market surveillance activities, including adverse event reporting, trend analysis, and implementation of corrective and preventive actions. We help manufacturers establish robust post-market surveillance systems to monitor the safety and performance of their devices effectively.
Regulatory Liaison: As part of our PRRC service, we serve as a dedicated point of contact for regulatory authorities, notified bodies, and other stakeholders. We represent our clients in regulatory interactions, ensure timely submission of regulatory documentation, and address any regulatory queries or concerns.
Continuous Support: We provide ongoing support and guidance to manufacturers to ensure ongoing compliance with regulatory requirements. Our PRRCs stay abreast of changes in regulations and standards, proactively addressing any updates or amendments that may impact our clients’ products.

Our PRRC Service Process
- Enquire about our PRRC Service
- Set up a call to identify how much support is needed
- Quotation stage and acceptance
- Patient Guard is happy to sign an NDA for confidentiality
- Service agreement put in place
- Onboarding – Monthly fee support provided based on level agreed
- Flexibility should you need less hours or more hours in a particular period
Benefits of working with Patient Guard
- Patient Guard ensures a simple and streamlined process.
- Patient Guard offers cost-effective services.
- Our team consists of highly qualified and experienced regulatory experts.
- Rest assured your devices meet all regulatory requirements.
- Patient Guard is backed by professional indemnity insurance.
- Enjoy transparent pricing with no hidden fees.
Cost of the PRRC Service
Our flexible service provides a number of hours per month based on the size of your operation and the amount of work that is involved. Hours can be increased or decreased in any period to give you control, and ensure that money is being spent wisely and only on the actual amount of service that is required.
We wont tie you into needless set contracted hours like many other consultancies.

Getting Started with Patient Guards PRRC Service
Get in touch to learn more about our PRRC service. We offer a free consultation where you can meet our team, discuss the process in detail, and share information about your company and products. After the consultation, we’ll provide you with a personalized quote and answer any questions you may have before you make a decision to proceed with our service.
PRRC FAQs
If you are a Medical Device or IVD manufacturer and place CE marked products on the market under the MDR or IVDR then you are required to have a PRRC
Yes, if you are a micro or small enterprise with a turn over of less than 10 million Euros then you can outsource the PRRC Service. If you generate more revenue than 10 million Euros then you must directly employ the PRRC within your business.
As soon as you place any product on the market under the MDR or IVDR you must have a PRRC in place.
Tracey Slater, Cormed
Find out more about medical device compliance and regulations
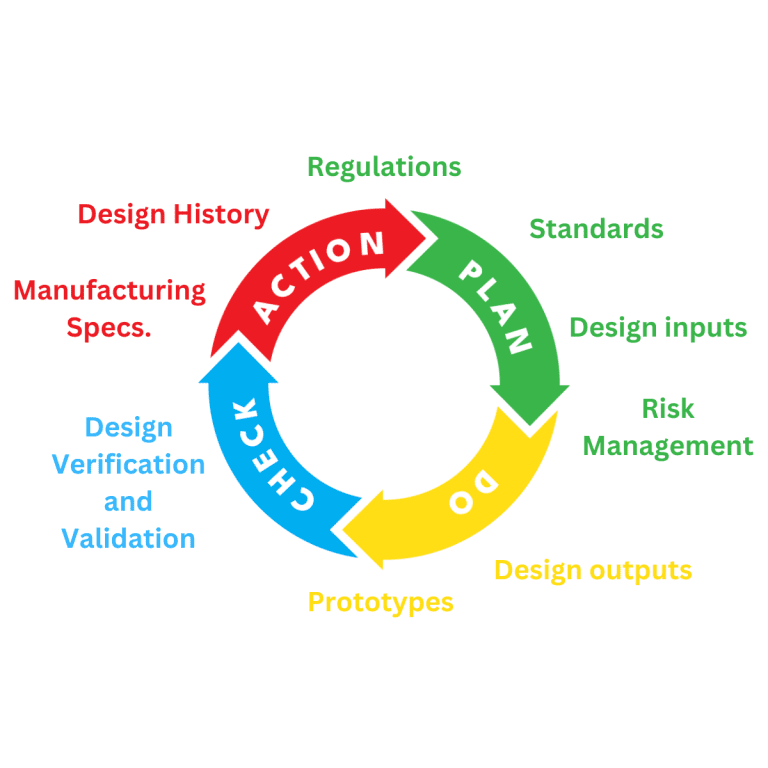
Medical Device Design and Development
Planning for the design and development of a medical device is a requirement of regulatory systems. All manufacturers of Medical

Understanding the core elements of Quality Management Systems
In today’s competitive marketplace, delivering high-quality products and services is essential for business success. A Quality Management System (QMS) is

5 Differences Between ISO 13485 & FDAs Medical Device QSR
Medical device manufacturers face a daunting task: navigating complex regulatory landscapes to ensure their products meet safety and quality standards.
ISO 13485 Standard – Quality Management System
The ISO 13485 standard was built around the structure of ISO 9001 which is a standard for Quality Management Systems


