MedTech Start-ups
Starting a medical device start-up company can be a stressful time, what with design and development activities, finding funding, ensuring you don’t run out of capital etc.
One very important area that is often not considered early enough is the regulatory side. Many medical device start-up companies think that the regulatory activities don’t begin until the device has been developed and they often don’t realise the cost involved in ensuring that the medical device meets the safety requirements of the medical device regulations globally.
It is important that medical device start-up companies look at regulatory support for medical device development from the offset.
This is because it is important to look at what the regulatory pathway looks like, how long things take and how much achieving the regulatory miles stones will cost, and of course ensuring you have enough budgeted for these costs.
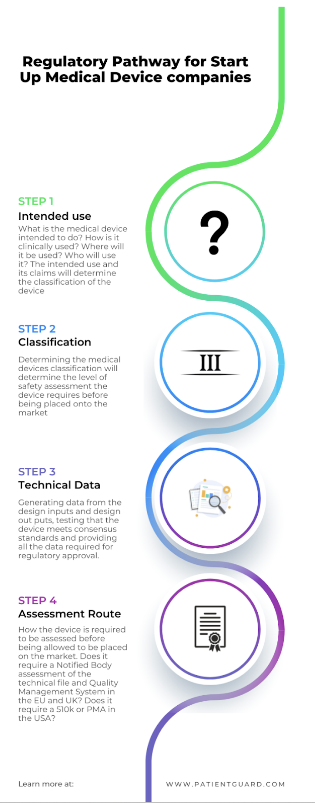
Device Concept
When an idea for a medical device has been formed, this is the stage you start to map out the regulatory pathway.
The first thing a medical device start-up need to determine is if the product will be a medical device based on its intended use.
The second things you need to consider is what market the device will be placed on?
Once you know this, you will be able to identify which regulations will be applicable to your company and determine if the product is a medical device or not.
If for example, if you decide to enter the EU market first then you would need to review the Medical Device Regulations EU 2017/745 or In Vitro Diagnostic Regulations EU 2017/746 to assess based on their definition of a medical device, your device will fall under such a description.
If you decided to enter the USA market first you would need to find an equivalent medical device using the FDA classification database, and if no equivalent product can be found, you would need to determine from the FDA what their opinion is on the product.
Medical Device Classification
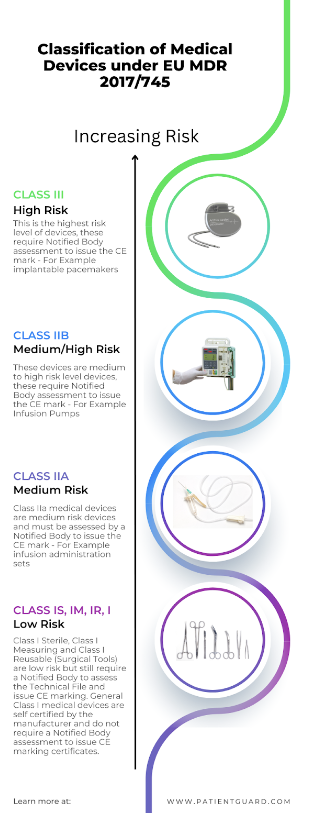
Once you have determined that your product will be a medical device then you need to classify the medical device start-up medical device. This is an important step in the regulatory process.
In the EU Annex VIII of both MDR EU 2017/745 and IVDR EU 2017/746 details the classification rules. These rules are used to determine the device classification and whether it will be a Class III, Class IIb, Class IIa, Class I (sterile), Class I (Measuring), Class I (reusable (surgical instrument)) or a Class I medical device.
In the USA the classification Database is used to find a product that is equivalent to your medical device, the classification database will identify what class of device (Class III, Class II or Class I) it will be and what the conformity route will be to place the product on the market. This could be 510k exempt for low risk devices, or requires a 510k for assessment by the FDA before being approved for sale. If no equivalent device can be identified then it is likely a PMA (Pre-Market Authorisation) submission would be required for the FDA to determine the classification and approve the device as safe to be placed on the market.
Technical Data
Europe Requirements
Evidence that your medical device meets the regulatory requirements is required for all medical device market jurisdictions. This evidence is collated and presented in the form of a Technical File.
The technical file will need to be presented in a particular format depending on the requirements of the regulations.
For example, in the EU a technical file needs to be set out in the following heading format.
- Device Description & Specification
- Device Description
- Device Names
- General Device Description
- UDI-DI
- Intended use
- Intended purpose
- Intended user
- Indications for use
- Intended Environment/Settings for use
- Contraindications
- Principles for operation
- Rationale of the product as a medical device
- Risk classification
- Novel features
- Accessories
- Configurations or variants
- Functional elements
- Raw materials
- Technical Specifications
- Previous or similar generations of device
- Information to be provided by the manufacturer.
- Labelling
- Instructions for use
- Design and manufacturing information
- Design stages
- Manufacturing processes
- Design and manufacturing sites
- Bill of Materials
- Supplier Quality Agreements
- Design Specifications
- Design History
- Material Safety Datasheets
- Design Drawings
- General safety and performance requirements
- GSPR Checklist
- Risk management
- Risk Management Plan
- Risk Identification
- Risk Evaluation and control measures
- Risk / Benefit analysis
- Residual Risk acceptability
- Verification and Validation
- Product Validation
- Packaging Validation
- Sterilisation Validation
- Software Validation
- Biological Evaluation
- Usability Engineering
- Clinical Evaluation
- Post Market Surveillance
- PMS Plan
- PMCF Plan
- PMS Reports or PSURs
- PMCF Reports
FDA Requirements
The FDA has a similar requirement for data to be presented in a particular format for example in a 510k submission.
Knowing what data you will need to generate specifically for your medical device is key to understanding the length of time it will take and the implicated costs, for example if your medical device has direct or indirect patient/user contact then Biological Evaluation will be required. If your medical device has a completely new clinical indication never seen on the market before or a new clinical indication to an existing medical device then you may need to perform clinical trials to generate enough clinical data to go into your clinical evaluation to prove clinical safety.
Conformity Assessment Route
The conformity assessment is another area to consider in terms of cost and time. Knowing what type of conformity assessment is required will help you to budget and include in your design and development time frames.
For example in the EU if your medical device is a class III, Class IIb or Class IIa medical device, then you will need to contract an EU approved Notified Body to assess your technical file and your Quality Management System (QMS).
If your medical device is a class I sterile, class I measuring or class I reusable surgical instrument then a Notified Body is also required to assess the aspects such as sterility and measurement.
You can not place your devices on the EU market before you receive your CE mark certification from the Notified Body.
Notified Body assessments can take a long time due to the current shortage of notified bodies within the EU and the significant back log of currently approved Notified Bodies. Therefore, it is important to engage with a Notified Body as soon as possible to build in to your time scale.
For Class I medical devices, in the EU these are manufacturer self-certified medical devices, however they need to follow the same principles such as device classification establishment, technical data and have a quality management system in place. However, they do not need a Notified Body to issue a CE mark certificate. However, they must draw up a declaration of conformity and register with a regulatory authority in the member state that the business is registered in.
It’s also important to remember that Notified Body Audits happen every year and so there will also be costs to consider for this.
In the USA it is a similar process however the FDA review the technical data during the submission process rather than a Notified Body.
Device Registration
Both in the EU and the USA all medical devices are required to be registered with a regulatory authority. In the USA this is the FDA. In the EU it is the member states regulatory authority of where the business is registered.
If the manufacturer (or person placing the medical device on the market) is not based in the USA for the purposes of FDA registration or in the EU for the purposes of CE mark device registration then the manufacturer is required to appoint a representative who is registered in the jurisdiction. The representative will register the devices on behalf of the foreign manufacturer.
This again is important in understanding what costs are involved in registration. In the USA medical devices incur registration fees which are required to be paid every year.
The Regulatory Road Map
In summary when starting a medical device company, its really important to map out all the regulatory requirement early on. This will save you countless time, costs, and resource later on. Not doing this can be a costly mistake, and a large number of medical device startups who don’t plan for this end up either going into administration or having to have subsequent funding rounds eroding equity in the company or burdening it with debt that will ultimately result in its collapse. With so many interesting new medical device ideas out there, it would be a shame to loose out on a potential medical device that could be life changing to its users.
Always use professional advice when looking for regulatory support. Patient Guard has a wealth of experience working with medical device start ups and can help you from start to finish with your medical device regulatory responsibilities.





