Medical Device Regulation and the future of the medical device industry
In an era marked by unprecedented advancements in healthcare technology, the medical device industry stands as a beacon of innovation. The development of cutting-edge medical devices has transformed the way healthcare is delivered, offering more precise diagnostics, improved treatments, and enhanced patient outcomes. In this blog, we will explore the biggest medical device companies in the world in 2023, the growth of this industry, the crucial role of medical device regulation, and how companies like Patient Guard are aiding in compliance and patient safety.
The Growing Medical Device Industry
The medical device industry has witnessed remarkable growth in recent years, and its expansion shows no signs of slowing down. With an aging population, increasing prevalence of chronic diseases, and a growing demand for personalized healthcare solutions, the global medical device market is projected to reach a staggering $603.5 billion by 2026, according to market research firm Grand View Research.
Several factors contribute to this growth:
- Technological Advancements: Breakthroughs in materials science, miniaturization, and connectivity have enabled the development of highly sophisticated medical devices, including wearable health tech, robotic surgery systems, and diagnostic imaging equipment.
- Demand for Minimally Invasive Procedures: Patients and healthcare providers alike are seeking less invasive treatment options, driving the demand for innovative medical devices that enable quicker recovery and reduced hospital stays.
- Global Health Challenges: The COVID-19 pandemic shed light on the importance of medical devices such as ventilators, diagnostic kits, and telemedicine tools, further accelerating the industry’s growth.
The Need for Medical Device Regulation
With innovation comes responsibility, especially in an industry where lives are at stake. The medical device sector is subject to strict regulatory oversight in many countries. These regulations are designed to ensure that devices are safe, effective, and meet quality standards. Key reasons for regulation in the medical device industry include:
- Patient Safety: Regulations safeguard patients from potentially harmful or ineffective devices. They set standards for design, manufacturing, labeling, and post-market surveillance.
- Efficacy: Regulations require manufacturers to provide evidence of a device’s effectiveness through clinical trials and rigorous testing, ensuring that patients receive treatments that work.
- Quality Control: Regulations mandate quality management systems to maintain consistency in manufacturing processes and prevent defects.
Patient Guard: Enhancing Medical Device Regulation Compliance
Regulatory compliance can be complex, time-consuming, and costly for medical device companies. This is where companies like Patient Guard come into play. Patient Guard is a technology platform that offers a range of services to help medical device companies navigate the regulatory landscape and prioritize patient safety. Here’s how it can assist:
- Regulatory Guidance: Patient Guard provides up-to-date information on medical device regulation changes and best practices, helping companies stay ahead of compliance requirements.
- Quality Management: The platform assists in establishing and maintaining quality management systems in line with industry standards.
- Post-Market Surveillance: Patient Guard helps companies monitor the performance of their devices in the market, ensuring that any issues are promptly addressed.
The Big Four: Leading Medical Device Companies in 2023
Let’s take a look at the four largest medical device companies in the world in 2023, according to the Fortune 500:
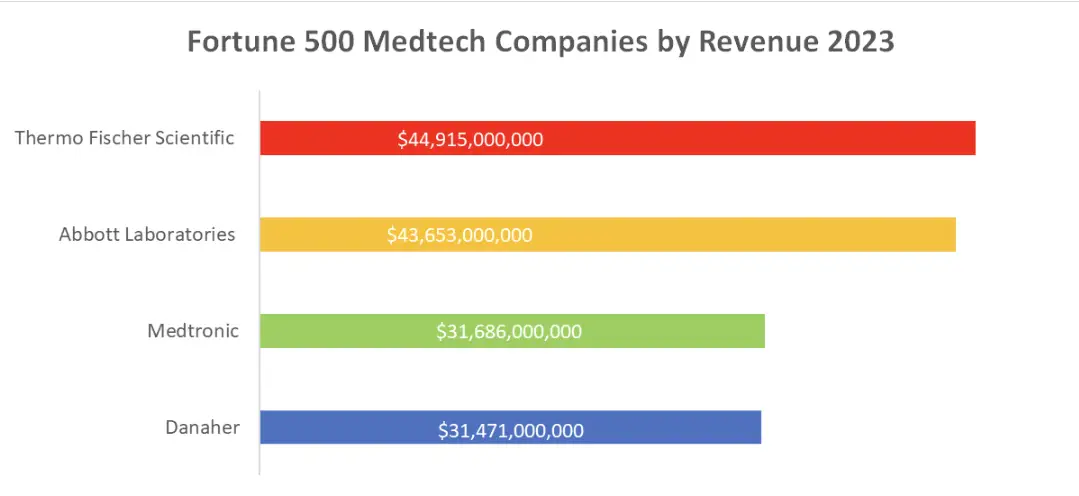
- Thermo Fischer Scientific: While primarily known for its scientific instruments and laboratory equipment, Thermo Fischer Scientific also produces a wide range of medical devices, including diagnostic tools and equipment for life sciences research.
- Abbott Laboratories: Abbott is a global healthcare company specializing in diagnostics, medical devices, nutrition, and branded generic pharmaceuticals. They are known for their cardiovascular and diabetes care devices.
- Medtronic: Medtronic is a pioneer in medical technology, with a focus on devices related to chronic diseases. They produce products ranging from pacemakers and insulin pumps to spinal implants.
- Danaher: Danaher’s Life Sciences segment includes a diverse array of medical devices, diagnostic equipment, and dental products. Their businesses span a wide range of healthcare applications.
Summary
In conclusion, the medical device industry is not only growing but evolving at a remarkable pace, driven by technological advancements and increasing healthcare demands. Medical Device Regulation remains a critical aspect of this industry, ensuring that innovation is balanced with patient safety and effectiveness. Companies like Patient Guard play a vital role in assisting medical device companies to navigate this complex regulatory landscape, fostering compliance and promoting patient well-being. As we move forward, the collaboration between innovation and regulation will continue to shape the future of healthcare and improve the lives of patients worldwide.
FAQs
Key trends influencing the medical device industry include:
- Digital Health Integration: Growth in telemedicine, wearable devices, and Software as a Medical Device (SaMD).
- Artificial Intelligence (AI): AI-driven diagnostics, personalized treatment, and predictive analytics.
- 3D Printing: Customized implants, prosthetics, and surgical tools.
- Minimally Invasive Devices: Advanced robotics and nanotechnology for less invasive procedures.
- Sustainability: Focus on eco-friendly materials and reducing medical waste.
Why it matters: These advancements promise improved patient outcomes, but they also introduce new regulatory challenges.
Regulatory bodies are updating frameworks to keep pace with innovation:
- EU MDR and IVDR: Emphasize stricter clinical evidence, post-market surveillance, and software regulation.
- FDA Guidance: Expanded focus on AI, machine learning, and digital health tools.
- Harmonized Standards: Adoption of globally recognized standards like ISO 13485 and IEC 62304.
- Adaptive Regulations: Frameworks evolving to address real-time updates for AI-driven devices.
Key insight: Staying informed about these regulatory changes is critical for timely market access.
AI and machine learning will revolutionize medical devices by enabling:
- Faster Diagnoses: AI-powered tools for imaging, pathology, and genetic analysis.
- Predictive Analytics: Anticipating patient needs and health outcomes.
- Personalized Medicine: Tailoring treatments based on individual data.
- Device Automation: Smarter, self-regulating devices for improved efficiency.
Pro tip: Developers must address transparency, bias, and validation challenges to ensure regulatory approval of AI-enabled devices.
The push for sustainability will influence future regulations in several ways:
- Material Selection: Encouraging biodegradable or recyclable materials.
- Energy Efficiency: Requirements for low-power consumption in electronic devices.
- Lifecycle Management: Regulatory focus on reuse, repair, and recycling.
- Carbon Footprint Monitoring: Expect frameworks to incorporate sustainability metrics.
Key takeaway: Sustainable design will become a regulatory expectation, not just a competitive advantage.
Global harmonization aims to streamline regulations, enabling manufacturers to access multiple markets with fewer barriers:
- IMDRF Initiatives: Promoting aligned standards for device safety and efficacy.
- Mutual Recognition Agreements (MRAs): Agreements between countries (e.g., EU-US) to reduce duplicative regulatory reviews.
- Digital Tools: Standardized databases like EUDAMED improve transparency and traceability.
Key insight: Harmonization simplifies compliance but requires manufacturers to stay up to date with evolving global standards.
Patient Guard provides comprehensive support for navigating future regulatory challenges, including:
- Preparing for AI and software-specific regulations like IEC 62304 and FDA AI/ML guidance.
- Assisting with compliance for digital health and wearable devices.
- Ensuring sustainability initiatives align with emerging regulatory trends.
- Guiding market entry with expertise in global harmonization frameworks like MDR, IVDR, and FDA regulations.
Why choose Patient Guard: With a proven track record in innovation-focused compliance, we help you stay ahead in an evolving regulatory landscape.




