In an era marked by unprecedented advancements in healthcare technology, the medical device industry stands as a beacon of innovation. The development of cutting-edge medical devices has transformed the way healthcare is delivered, offering more precise diagnostics, improved treatments, and enhanced patient outcomes. In this blog, we will explore the biggest medical device companies in the world in 2023, the growth of this industry, the crucial role of regulation, and how companies like Patient Guard are aiding in compliance and patient safety.
The Growing Medical Device Industry
The medical device industry has witnessed remarkable growth in recent years, and its expansion shows no signs of slowing down. With an aging population, increasing prevalence of chronic diseases, and a growing demand for personalized healthcare solutions, the global medical device market is projected to reach a staggering $603.5 billion by 2026, according to market research firm Grand View Research.
Several factors contribute to this growth:
- Technological Advancements: Breakthroughs in materials science, miniaturization, and connectivity have enabled the development of highly sophisticated medical devices, including wearable health tech, robotic surgery systems, and diagnostic imaging equipment.
- Demand for Minimally Invasive Procedures: Patients and healthcare providers alike are seeking less invasive treatment options, driving the demand for innovative medical devices that enable quicker recovery and reduced hospital stays.
- Global Health Challenges: The COVID-19 pandemic shed light on the importance of medical devices such as ventilators, diagnostic kits, and telemedicine tools, further accelerating the industry’s growth.
The Need for Regulation
With innovation comes responsibility, especially in an industry where lives are at stake. The medical device sector is subject to strict regulatory oversight in many countries. These regulations are designed to ensure that devices are safe, effective, and meet quality standards. Key reasons for regulation in the medical device industry include:
- Patient Safety: Regulations safeguard patients from potentially harmful or ineffective devices. They set standards for design, manufacturing, labeling, and post-market surveillance.
- Efficacy: Regulations require manufacturers to provide evidence of a device’s effectiveness through clinical trials and rigorous testing, ensuring that patients receive treatments that work.
- Quality Control:Regulations mandate quality management systems to maintain consistency in manufacturing processes and prevent defects.
Patient Guard: Enhancing Regulatory Compliance
Regulatory compliance can be complex, time-consuming, and costly for medical device companies. This is where companies like Patient Guard come into play. Patient Guard is a technology platform that offers a range of services to help medical device companies navigate the regulatory landscape and prioritize patient safety. Here’s how it can assist:
- Regulatory Guidance: Patient Guard provides up-to-date information on regulatory changes and best practices, helping companies stay ahead of compliance requirements.
- Quality Management: The platform assists in establishing and maintaining quality management systems in line with industry standards.
- Post-Market Surveillance: Patient Guard helps companies monitor the performance of their devices in the market, ensuring that any issues are promptly addressed.
The Big Four: Leading Medical Device Companies in 2023
Let’s take a look at the four largest medical device companies in the world in 2023, according to the Fortune 500:
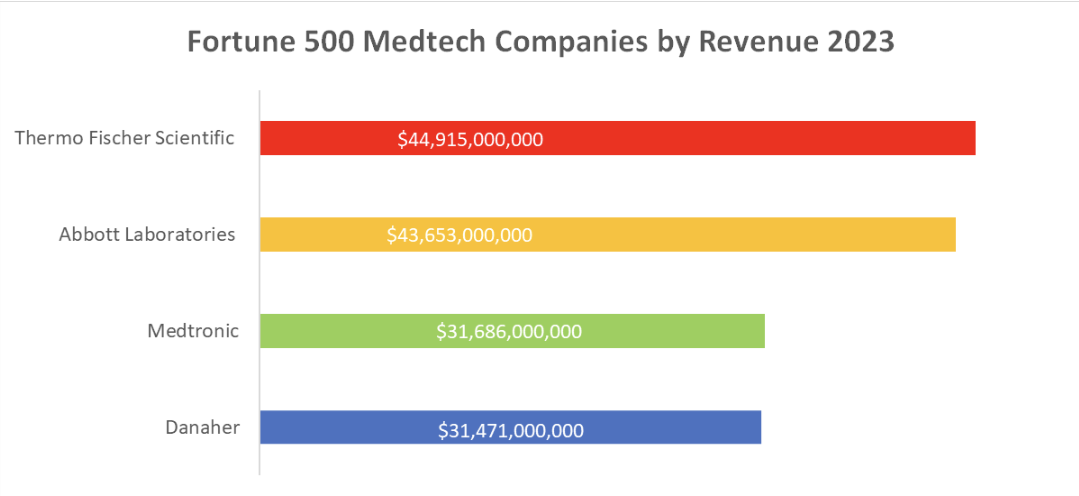
- Thermo Fischer Scientific: While primarily known for its scientific instruments and laboratory equipment, Thermo Fischer Scientific also produces a wide range of medical devices, including diagnostic tools and equipment for life sciences research.
- Abbott Laboratories: Abbott is a global healthcare company specializing in diagnostics, medical devices, nutrition, and branded generic pharmaceuticals. They are known for their cardiovascular and diabetes care devices.
- Medtronic: Medtronic is a pioneer in medical technology, with a focus on devices related to chronic diseases. They produce products ranging from pacemakers and insulin pumps to spinal implants.
- Danaher: Danaher’s Life Sciences segment includes a diverse array of medical devices, diagnostic equipment, and dental products. Their businesses span a wide range of healthcare applications.
In conclusion, the medical device industry is not only growing but evolving at a remarkable pace, driven by technological advancements and increasing healthcare demands. Regulation remains a critical aspect of this industry, ensuring that innovation is balanced with patient safety and effectiveness. Companies like Patient Guard play a vital role in assisting medical device companies to navigate this complex regulatory landscape, fostering compliance and promoting patient well-being. As we move forward, the collaboration between innovation and regulation will continue to shape the future of healthcare and improve the lives of patients worldwide.