Quality Management System
In today’s competitive marketplace, delivering high-quality products and services is essential for business success. A Quality Management System (QMS) is a structured framework that helps organizations ensure they meet customer requirements and enhance customer satisfaction. But what are the fundamental elements that constitute a robust QMS? In this blog, we will explore the basic components that form the backbone of effective quality management systems.

1. Quality Policy and Objectives
At the heart of any QMS is a clear and concise quality policy. This policy sets the overarching goals and commitments of the organization regarding quality. It should reflect the company’s vision, mission, and core values. Alongside the quality policy, specific, measurable quality objectives should be established. These objectives provide direction and benchmarks for evaluating performance.
2. Documented Information
A well-documented QMS ensures consistency and traceability. This includes maintaining detailed records of procedures, work instructions, and quality manuals. Proper documentation helps in standardizing processes, training new employees, and auditing the system. It also serves as evidence of compliance with regulatory and customer requirements.

3. Process Approach
A QMS is built around the process approach, which views activities as interrelated processes that function as a coherent system. This approach emphasizes understanding and managing processes to improve efficiency and achieve desired outcomes. By mapping out processes, organizations can identify areas for improvement and streamline operations.
4. Leadership & Commitment
Effective leadership is crucial for the success of a QMS. Top management must demonstrate a strong commitment to quality by providing resources, setting strategic direction, and fostering a culture of continuous improvement. Leadership involvement ensures that quality objectives align with business goals and that the entire organization is motivated to achieve them.
5. Customer Focus
A key principle of quality management is maintaining a strong customer focus. This means understanding customer needs and expectations, delivering products and services that meet or exceed those expectations, and continually seeking feedback to enhance customer satisfaction. A QMS should facilitate processes that prioritize customer requirements and measure satisfaction levels.

6. Risk-Based Thinking
Incorporating risk-based thinking into a QMS helps organizations proactively identify and address potential issues that could impact quality. This involves assessing risks and opportunities within processes and making informed decisions to mitigate risks. By managing risks effectively, companies can prevent problems before they occur and seize opportunities for improvement.
7. Performance Evaluation
Regular monitoring and measurement of performance are essential to ensure the QMS is effective and achieving its objectives. This includes conducting internal audits, management reviews, and analyzing key performance indicators (KPIs). Performance evaluation provides valuable insights into areas that need attention and helps in making data-driven decisions for continuous improvement.

8. Continuous Improvement
Continuous improvement is a core tenet of quality management. A QMS should foster a culture where employees are encouraged to suggest improvements and innovation is promoted. Tools like PDCA (Plan-Do-Check-Act) cycles and Six Sigma methodologies can be used to systematically enhance processes and drive quality enhancements.
9. Training and Competence
Ensuring that employees are competent and adequately trained is vital for the success of a QMS. This involves identifying training needs, providing necessary training, and evaluating its effectiveness. Competent employees are better equipped to perform their tasks accurately and contribute to the organization’s quality objectives.

10. Supplier Quality Management
Suppliers play a significant role in the quality of products and services. A robust QMS includes processes for selecting, evaluating, and managing suppliers to ensure they meet quality standards. Building strong relationships with suppliers and involving them in the quality process can lead to better material quality and reliability.
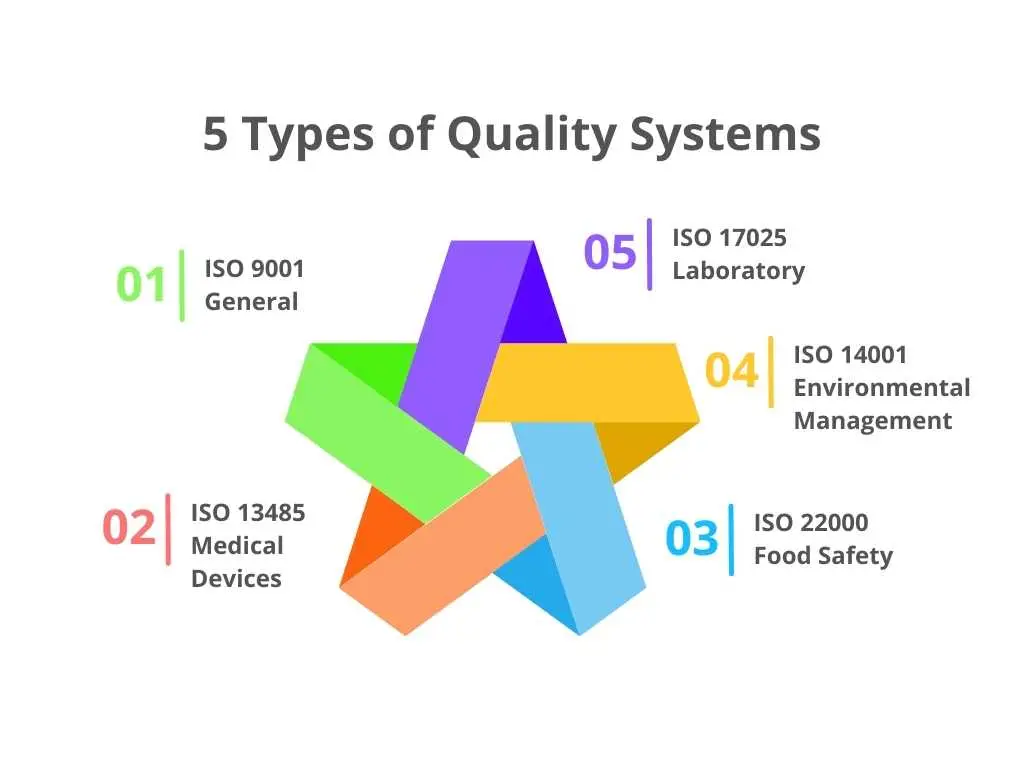
ISO 9001: The General Quality Management System Standard
ISO 9001 is the most widely recognized and implemented quality management standard globally. It provides a framework for organizations of all sizes and industries to ensure they meet customer and regulatory requirements while continually improving their processes. Unlike sector-specific standards, ISO 9001 is designed to be applicable to any organization that wants to establish a QMS.
ISO 9001 focuses on several key principles, including customer satisfaction, leadership, engagement of people, process approach, improvement, evidence-based decision making, and relationship management. By adhering to ISO 9001, organizations can enhance operational efficiency, reduce waste, and foster a culture of continuous improvement. The flexibility and broad applicability of ISO 9001 make it the go-to standard for organizations seeking to improve quality and gain a competitive edge in the market.
ISO 13485: Medical Device Quality Management System
For organizations involved in the design, production, and servicing of medical devices and in vitro diagnostics (IVDs), adhering to ISO 13485 is essential. ISO 13485 is an internationally recognized standard specifically designed for the medical device industry. It sets the criteria for a comprehensive quality management system that ensures medical devices are consistently produced to meet regulatory requirements and customer expectations.
ISO 13485 places a strong emphasis on risk management and the ability to maintain effective processes throughout the product lifecycle. This includes stringent requirements for documentation, process controls, and validation procedures. Compliance with ISO 13485 is not just about meeting regulatory mandates but also about safeguarding patient safety and enhancing the reliability and performance of medical devices. Implementing a QMS based on ISO 13485 can help organizations streamline their processes, improve product quality, and build trust with stakeholders in the healthcare industry.
Other Well-Known Quality Management System Standards
In addition to ISO 9001 and ISO 13485, several other QMS standards are widely recognized and implemented across various industries. These standards cater to specific sectors, addressing their unique quality requirements and regulatory challenges.
ISO/TS 16949:
This standard is designed for the automotive industry and focuses on continuous improvement, defect prevention, and the reduction of variation and waste in the supply chain. It integrates the requirements of ISO 9001 with additional automotive industry-specific requirements.
ISO 22000:
This standard is specific to the food safety management systems. It addresses the needs of organizations in the food chain, from farmers to food services, to ensure food safety and hygiene throughout the production and supply process.
ISO 17025:
This standard applies to laboratories and specifies the general requirements for the competence to carry out tests and/or calibrations. It ensures that laboratories produce precise and accurate data.
AS9100:
Tailored for the aerospace industry, AS9100 incorporates the requirements of ISO 9001 with additional aerospace sector-specific standards. It aims to ensure the safety, reliability, and quality of products and services within the aerospace sector.
ISO 14001:
While not a QMS standard per se, ISO 14001 focuses on environmental management systems. It helps organizations improve their environmental performance through more efficient use of resources and reduction of waste, which often complements quality management efforts.
Summary
Implementing an effective Quality Management System is a comprehensive process that requires commitment and continuous effort. By focusing on these core elements, organizations can build a strong foundation for delivering high-quality products and services, achieving customer satisfaction, and sustaining business success. Remember, quality is not just a goal but a journey of constant improvement and excellence.
FAQs
A Quality Management System (QMS) is a structured framework of policies, processes, and procedures designed to ensure that medical devices meet regulatory requirements and consistently deliver safe, effective, and high-quality products.
Why it matters: A QMS is mandatory for medical device manufacturers to comply with global standards like ISO 13485 and regulations such as EU MDR 2017/745 and FDA 21 CFR Part 820.
ISO 13485 is an internationally recognized standard that specifies QMS requirements for medical device design, development, production, installation, and servicing. It aligns with regulatory needs in key markets, including the EU, US, and UK.
Key insight: ISO 13485 certification demonstrates a manufacturer’s commitment to quality and regulatory compliance, facilitating market access.
A robust QMS for medical devices typically includes:
- Document Control: Managing policies, procedures, and records.
- Risk Management: Integrating risk assessments following ISO 14971.
- Design Control: Documenting the design and development process.
- Supplier Management: Ensuring suppliers meet quality standards.
- Production and Process Control: Maintaining consistent manufacturing processes.
- Post-Market Surveillance (PMS): Monitoring device performance after market launch.
Pro tip: Tailor your QMS to your device’s risk classification and market requirements.
Yes, a QMS is mandatory for regulatory approval in most major markets:
- CE Marking (EU): The EU MDR and IVDR require a QMS aligned with ISO 13485.
- FDA Approval (US): The FDA mandates QMS compliance under 21 CFR Part 820.
- UKCA Marking (UK): A QMS is required under the UK Medical Device Regulations.
Key takeaway: A QMS is the foundation for regulatory submissions and audits.
Manufacturers often face challenges such as:
- Regulatory Complexity: Navigating evolving global regulations.
- Resource Constraints: Lack of dedicated personnel or expertise.
- Documentation Overload: Managing extensive documentation requirements.
- Audit Readiness: Preparing for inspections and regulatory audits.
Solution: Partnering with a QMS expert or consultant can simplify implementation and ensure compliance.
Absolutely! Patient Guard offers end-to-end QMS services, including:
- Developing ISO 13485-compliant QMS tailored to your needs.
- Conducting gap analyses to identify areas for improvement.
- Preparing for CE marking, FDA approval, or UKCA certification audits.
- Providing ongoing support for QMS updates and maintenance.
Why choose Patient Guard: With experience helping over 500 manufacturers, we streamline QMS implementation to ensure regulatory compliance and market readiness.




