Extractables and Leachables testing in Medical Devices
In medical device design and development and manufacturing, ensuring the safety and biocompatibility of products is paramount. As part of the biological evaluation process, extractables and leachables (E&L) testing plays a crucial role. This testing ensures that any chemicals that might migrate from the device materials into the body do not pose a risk to patients. The ISO 10993 standard series provides comprehensive guidelines for the biological evaluation of medical devices, including protocols for E&L testing. In this blog, we will delve into the importance of extractables and leachables testing, its procedures, and its relevance within the ISO 10993 framework.
What Are Extractables and Leachables?
Extractables are chemicals that can be extracted from device materials under aggressive laboratory conditions, such as high temperature or solvent exposure. These conditions are designed to simulate the worst-case scenario of chemical release from the device.
Leachables, on the other hand, are chemicals that can migrate from device materials under normal usage conditions. This might include exposure to bodily fluids, contact with medications, or prolonged storage.

Why Is Extractables & Leachables Testing Important?
E&L testing is essential for several reasons:
- Patient Safety: It identifies potentially harmful chemicals that could leach from the device and cause adverse reactions in patients.
- Regulatory Compliance: Many regulatory bodies, including the FDA and EMA, require comprehensive E&L testing as part of the approval process for medical devices.
- Product Quality: Ensuring that no harmful substances leach from the device helps maintain high product quality and reliability.
- Reduction of Animal Testing: By demonstrating that chemical levels are below toxicological concern, E&L testing can reduce or eliminate the need for animal testing.
The Role of ISO 10993
ISO 10993 is a set of international standards for the biological evaluation of medical devices. It provides a risk management framework to identify potential biological hazards associated with medical devices. E&L testing falls under ISO 10993-12 and ISO 10993-18, which cover sample preparation and chemical characterization, respectively.
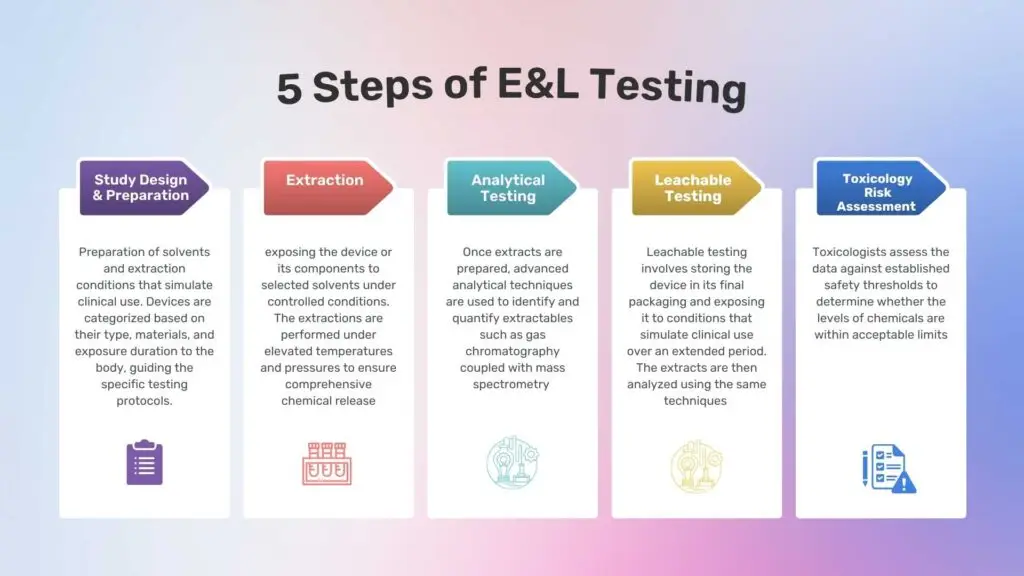
The E&L Testing Process
1. Study Design and Preparation
The first step involves designing the study to reflect the intended use of the medical device. This includes selecting appropriate solvents and extraction conditions that simulate clinical use. Devices are categorized based on their type, materials, and exposure duration to the body, guiding the specific testing protocols.
2. Extraction
This phase involves exposing the device or its components to selected solvents under controlled conditions. Common solvents include water, alcohols, and organic solvents, chosen to mimic the device’s clinical environment. The extractions are performed under elevated temperatures and pressures to ensure comprehensive chemical release.
3. Analytical Testing
Once extracts are prepared, advanced analytical techniques are used to identify and quantify extractables. These techniques include:
- Gas Chromatography-Mass Spectrometry (GC-MS): Ideal for volatile and semi-volatile organic compounds.
- Liquid Chromatography-Mass Spectrometry (LC-MS): Effective for non-volatile and polar compounds.
- Inductively Coupled Plasma-Mass Spectrometry (ICP-MS): Used for detecting metal impurities.
4. Leachable Testing
Leachable testing involves storing the device in its final packaging and exposing it to conditions that simulate clinical use over an extended period. The extracts are then analyzed using the same techniques to identify any chemicals that have leached under normal usage conditions.
5. Toxicological Risk Assessment
The final step is a toxicological risk assessment, where identified extractables and leachables are evaluated for their potential health risks. Toxicologists assess the data against established safety thresholds to determine whether the levels of chemicals are within acceptable limits.
ISO 10993-17 and ISO 10993-18: Key Components
ISO 10993-17: Toxicological risk assessment of medical device constituents
ISO 10993-17 provides guidance on determining allowable limits for leachable substances from medical devices. It focuses on assessing the risk posed by chemical substances that could migrate from device materials into the patient’s body. This standard involves toxicological risk assessment and establishing safety thresholds, ensuring that the levels of leachables are within acceptable limits for patient safety. It helps manufacturers in:
- Determining acceptable intake levels of chemical substances.
- Evaluating potential health risks associated with exposure to leachables.
- Setting limits that ensure patient safety over the device’s lifespan.
ISO 10993-18: Chemical Characterization of Materials
ISO 10993-18 outlines the procedures for the chemical characterization of materials used in medical devices. It provides detailed guidance on identifying and quantifying the chemical constituents of device materials. This standard is crucial for understanding the chemical composition of the materials and assessing their potential to release harmful substances. Key aspects include:
- Identifying all chemical constituents of the device materials.
- Quantifying the amount of each constituent.
- Evaluating the potential for these chemicals to be released under clinical conditions.
Importance of E&L Testing for Medical Devices Containing Plastics, Metals, and Ceramics
Medical devices are often composed of various materials, including plastics, metals, and ceramics, each of which can pose unique challenges in terms of chemical leaching.
Plastics:
Plastics are widely used in medical devices due to their versatility and cost-effectiveness. However, they can contain additives, plasticizers, and residual monomers that might leach out and cause toxicity or adverse reactions in patients. E&L testing helps identify these potential contaminants, ensuring the safety and compatibility of plastic-based devices.
Metals:
Metals are used in implants, surgical instruments, and various other medical devices. They can release metal ions, such as nickel, chromium, and cobalt, which may cause allergic reactions or cytotoxic effects. E&L testing of metal components ensures that any leached metal ions are within safe limits, preventing potential harm to patients.
Ceramics:
Ceramic materials are employed in dental implants, joint replacements, and other medical applications for their biocompatibility and durability. Despite their inert nature, ceramics can still release trace elements or undergo degradation under certain conditions. E&L testing ensures that ceramic-based devices do not leach harmful substances over time.
Reducing Animal Testing Through E&L Testing
One of the significant benefits of E&L testing is its potential to reduce the need for animal testing. When E&L testing demonstrates that the levels of extractables and leachables are below toxicological concern, it provides sufficient evidence that the device is unlikely to cause harm to patients. This data can be used to support biocompatibility claims, potentially satisfying regulatory requirements without the need for additional animal studies.

By focusing on chemical characterization and risk assessment, companies can:
- Avoid unnecessary animal testing by demonstrating safety through rigorous analytical methods.
- Meet ethical and regulatory standards for reducing animal use in testing.
- Accelerate the approval process by providing clear, documented evidence of safety.
Challenges in E&L Testing
While E&L testing is critical, it comes with its challenges:
- Complexity of Materials: Medical devices often comprise multiple materials, making it difficult to predict and analyze all potential extractables and leachables.
- Analytical Sensitivity: Detecting and quantifying trace levels of chemicals requires highly sensitive and precise analytical methods.
- Regulatory Expectations: Keeping up with evolving regulatory requirements and expectations can be demanding.
Summary
Extractables and leachables testing is a vital component of the biological evaluation of medical devices, ensuring patient safety and regulatory compliance. Adhering to the guidelines set forth in ISO 10993, including ISO 10993-17 and ISO 10993-18, helps manufacturers systematically assess and mitigate risks associated with chemical leachables from medical devices. By understanding and implementing robust E&L testing protocols, manufacturers can ensure their devices are safe, reliable, and ready for clinical use, ultimately protecting patient health, enhancing the quality of healthcare, and reducing the reliance on animal testing.
Patient Guard Ltd: Your Partner in Extractables and Leachables Testing
Patient Guard Ltd offers comprehensive Extractables and Leachables (E&L) testing services for medical devices, ensuring that all potential risks and regulatory requirements are meticulously addressed. By choosing Patient Guard, customers gain peace of mind knowing that our expert team considers every aspect of medical device safety and compliance in the design of E&L testing protocols. We pride ourselves on delivering cost-effective and efficient solutions, helping you meet industry standards while maintaining high product quality. Trust Patient Guard Ltd to safeguard your products and, ultimately, the patients who rely on them.
Related Services by Patient Guard:
FAQs
Extractables and leachables (E&L) testing evaluates the presence of chemical substances that may migrate from a medical device’s materials into the patient’s body under normal use or accelerated conditions.
- Extractables: Compounds that can be extracted under exaggerated conditions (e.g., high heat or aggressive solvents).
- Leachables: Compounds that migrate from the device during actual use.
Why it matters: E&L testing ensures that devices do not release harmful substances, safeguarding patient safety and regulatory compliance.
E&L testing is critical to:
- Assess biocompatibility as part of ISO 10993 biological evaluations.
- Identify potential toxicological risks associated with device materials.
- Ensure compliance with global regulations such as EU MDR 2017/745, FDA guidelines, and ISO standards.
Key takeaway: E&L testing is essential for devices that directly or indirectly contact the patient, such as implants, catheters, and syringes.
The E&L testing process typically involves:
- Sample Preparation: Device materials are prepared for extraction using solvents and controlled conditions.
- Extractables Testing: Materials are exposed to exaggerated conditions to identify all potential chemical substances.
- Leachables Testing: Simulated use conditions are applied to identify chemicals that leach during normal use.
- Chemical Analysis: Techniques like GC-MS, LC-MS, and ICP-MS are used to detect and quantify substances.
- Toxicological Risk Assessment: Data is evaluated for safety implications.
Pro tip: A risk-based approach ensures testing focuses on critical device components.
E&L testing is required for:
- Implantable Devices: Pacemakers, stents, orthopedic implants.
- Drug-Device Combinations: Prefilled syringes, drug-eluting stents, transdermal patches.
- Devices with Fluid Pathways: IV sets, catheters, dialysis systems.
- Sterile Packaging: To ensure no harmful substances migrate into the device during storage.
Key insight: Any device that comes into prolonged contact with body tissues, fluids, or pharmaceuticals should undergo E&L testing.
Common challenges include:
- Complex Material Composition: Modern devices often use multi-layered or polymer-based materials.
- Trace Analysis: Detecting extremely low levels of chemicals requires advanced analytical techniques.
- Toxicological Assessment: Interpreting the safety of detected substances can be complex and requires expert input.
Solution: Work with specialized labs and regulatory experts to address these challenges effectively.
Yes! Patient Guard provides comprehensive support for E&L testing, including:
- Developing testing protocols aligned with ISO 10993 and regulatory requirements.
- Coordinating testing with accredited laboratories.
- Conducting toxicological risk assessments for extractables and leachables.
- Preparing reports for regulatory submissions, including CE marking and FDA approvals.
Why choose Patient Guard: With a proven track record in biological evaluation, we help manufacturers ensure their devices meet safety and regulatory standards efficiently.



