As we enter the new year, we begin planning for the upcoming year, contemplating the Regulatory and Quality Assurance requirements for Medical Devices and IVDs. In this newsletter, we provide updates from regulatory authorities and standards organizations, aiding in your planning for compliance activities.
The MHRA has unveiled a roadmap for future UK regulatory implementation, offering insights into their thought process on Medical Device and IVD Regulatory activities. The guidance outlines extended timeframes for accepting CE marked medical devices and IVDs in the UK market.
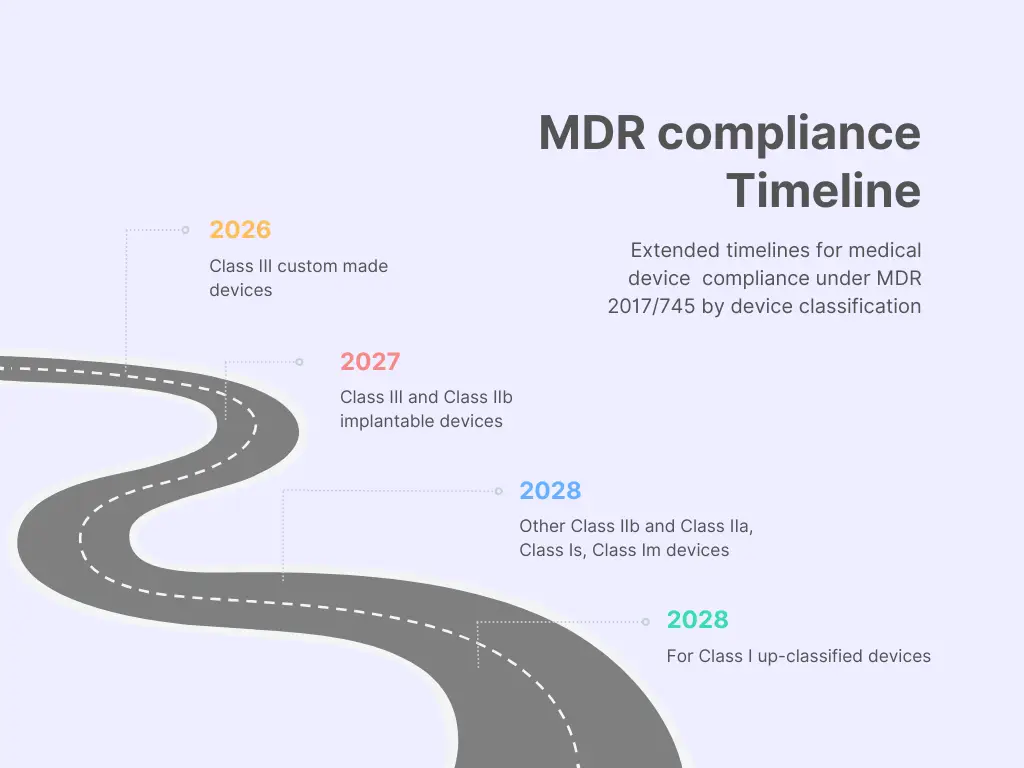
In the EU, it’s crucial to note the deadline for Manufacturers placing CE mark certified Medical Devices on the market. Those not yet transitioning to the EU MDR 2017/745 must have a contract with an EU-approved Notified Body for recertification by May 2024.